PHARMACEUTICAL MANUFACTURING SYSTEMS
Sterility Testing IsolatorThis isolator easily maintains advanced aseptic conditions for sterility testing by eliminating interference of operators. There is therefore no need for maintaining or running a cleanroom. This isolator also satisfies various regulatory requirements.

Incorporating a hydrogen peroxide decontamination system developed solely by Shibuya, this isolator realizes decontamination in a short time. The modular design of the isolator allows establishment of the aseptic testing environment which best fits your needs.
- The flexible modular design allows you to select the size optimum to your needs.
- Glove sizes and thicknesses can be selected from a wide variety.
- Connection with various components including a pass box and a RTP is possible.
- A variety of customizations is available.
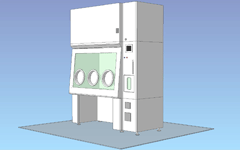
Built-in decontamination unit / 3 gloves
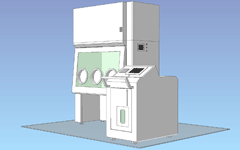
Movable decontamination unit / 3 gloves
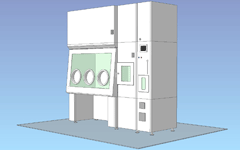
Pass box / Built-in decontamination unit / 3 gloves
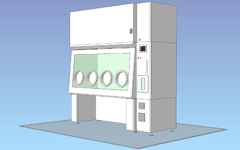
Built-in decontamination unit / 4 gloves
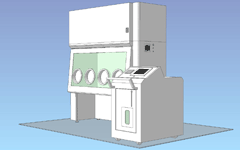
Movable decontamination unit / 4 gloves
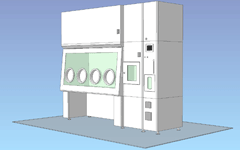
Pass box / Built-in decontamination unit / 4 gloves
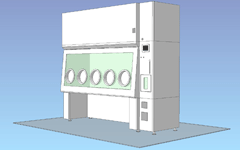
Built-in decontamination unit / 5 gloves
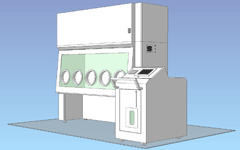
Movable decontamination unit / 5 gloves
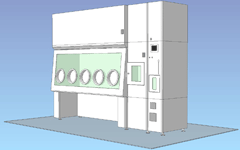
Pass box / Built-in decontamination unit / 5 gloves
- Inquiries
-
SHIBUYA CORPORATION
International Plant Sales Div.
Ko-58 Mameda-Honmachi, Kanazawa, Ishikawa 920-8681 JAPAN
Telephone : +81(76)262-1615 Fax : +81(76)223-1795
E-mail packaging@shibuya.co.jp