REGENERATIVE MEDICINE MANUFACTURING SYSTEMS
Application ExamplesProduction of bone marrow-derived stem cell-based therapies for liver regeneration
In this cell processing system, mononuclear cell components are separated from an autologous bone marrow fluid, and then concentrated and washed to manufacture bone marrow-derived stem cell products for liver regeneration. Also, in seeking less-invasive processes, a technique has been developed to proliferate stem cells derived from bone marrow fluid after extracting a small amount of bone marrow fluid from the patient. Bone marrow-derived stem cell products are manufactured for use in the treatment of the liver and other treatment applications currently in clinical study.
- Suitable for
- Autologous bone marrow cell fluid
- Culture vessel
- Blood bag
- Cell processing and manipulation
- Cell separation/cleansing

Autologous bone
marrow fluid
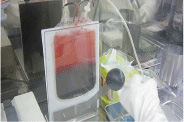
Manipulating cells

Cell processing isolator

"CPi" Cell Culture Isolator
[Yamaguchi Univ. /
Ube City MCC]

"CellPROi" Robotic Cell
Culture System
[Yamaguchi Univ. /
Ube City MCC]

Incubator
(Aseptically Connected
to Isolator,
VPHP Decontamination
Available)
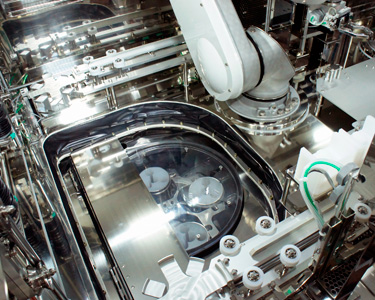
Built-in Centrifuge
(VPHP Decontamination
Available)
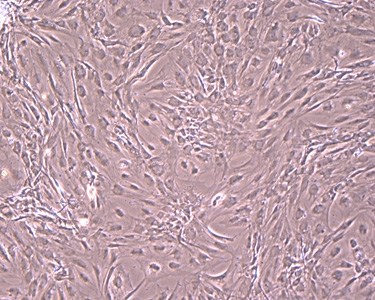
Cell Observation
inside Isolators
(Microscopic Image)
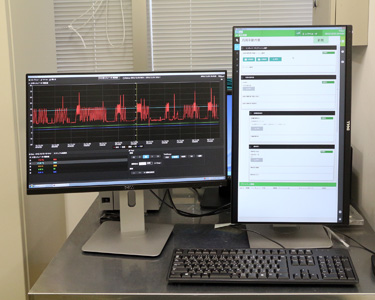
Manufacturing
Management System

Standard Operating
Procedure (SOP) Support
System
Cell Processing Facility/Factory Engineering Support
Environmental Monitoring System (Facility/Factory)
Environmental Monitoring System (Isolator)
Remote Maintenance System
Mass cultivation of adipose-derived stem cells and spheroid or 3D cell culture
In this system, cellular spheroids and stem cell constructs are manufactured after mass cultivating fat-derived stem cells. This equipment consists of an automated robotic cell culture system that performs a series of cell culture processing processes including cell medium exchange, subculturing, dispensation, and stem cell construct production.
Production of high-density mesenchymal stem cell, scaffold-free autologous constructs (HDMACs) for cartilage regeneration
- Suitable for
- Fat-derived stem cells (Adherent cells)
- Culture vessel
- 150 mm dish
96-well plate - Cell processing and manipulation
- Cell medium exchange / Subculturing
Cell observation
Dispensation
Production of stem cell constructs for transplant
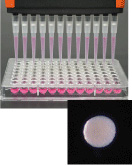
Cell mass

High-density mesenchymal stem cell,
scaffold-free autologous constructs (HDMACs) for cartilage regeneration produced from fat-derived stem cells
[Nakayama Laboratory, Saga University]

Robotic cell culture system

Robotic cell manipulation
Production of 3D constructs
This equipment performs 3D tissue/organ production by assembling cellular spheroids on to a needle block according to a program without using cell scaffold materials (scaffold-free).
- Suitable for
- Fat-derived stem cells
Fibroblasts
Endothelial cells
Smooth muscle cells
Hepatic cells, etc. - Culture vessel
- 96-well plate

Blood vessel shaped construct (Left: 3D data source) [Nakayama Laboratory, Saga Univ.]

Meniscus shaped construct (Left: 3D data source) [Nakayama Laboratory, Saga Univ.]

Bio 3D printer

Interior of the Bio 3D printer
Production of cell sheets
*Sterile environment & Production management system JST/FIRST Program
- Suitable for
- Fibroblasts
- Culture vessel
- Special container
- Cell processing and manipulation
- Isolation
↓
Mass cultivation
↓
Sheet layering

Myocardial regeneration sheet
Corneal regeneration sheet
Esophageal regeneration sheet
Periodontal regeneration sheet
Cartilage regeneration sheet

Cell sheet production system
- INQUIRES
-
SHIBUYA CORPORATION
Regenerative Medicine Manufacturing Systems Div. Sales Dept.
2-1 Hokuyodai, Kanazawa, Ishikawa 920-0177 JAPAN
Telephone : +81(0)76-257-8915 Fax : +81(0)76-257-8919
E-mail rm-info@shibuya.co.jp

