REGENERATIVE MEDICINE MANUFACTURING SYSTEMS
Robotic Cell Culture System "CellPROi"A ground-breaking automated cell culture system
This system consists of an isolator with a built-in two-armed robot that can be decontaminated using vaporized hydrogen peroxide. A sterile operation environment of a CPC and an automated cell culture and cell processing robot are integrated inside this compact isolator.

- Equipped with a two-armed robot that can be decontaminated using vaporized hydrogen peroxide
- Significant reduction in operator labor costs
- Steady culturing of consistent, high quality cells
- Humans, the primary contaminant source, are completely separated from cell culture operation
- No need for a CPC facility which requires rigorous training and contamination control
- An incubator can be mounted and removed aseptically making the system suitable for mass cell cultivation
- Robotic cell culture is reliable and more reproducible than that performed by the most experienced technician
- Can be operated manually and smoothly using the provided manual remote control system
- Dramatic reduction in initial costs due to the elimination of complex zoning required for a CPC facility
- Realizes low running costs by reducing validation, energy consumption and general operating costs and reducing risk of cell product loss due to contamination
- Has a built-in HYDEC model vaporized hydrogen peroxide decontamination system developed by unique Shibuya technology

Incubator (Aseptically Connected to Isolator, VPHP Decontamination Available)
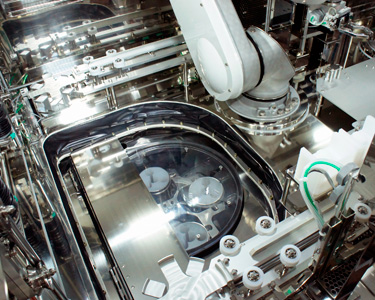
Built-in Centrifuge (VPHP Decontamination Available)
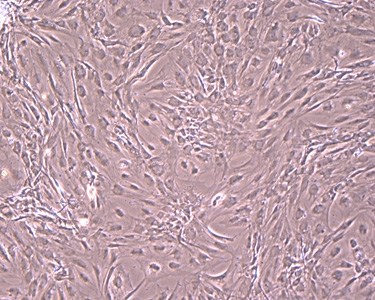
Cell Observation inside Isolators (Microscopic Image)
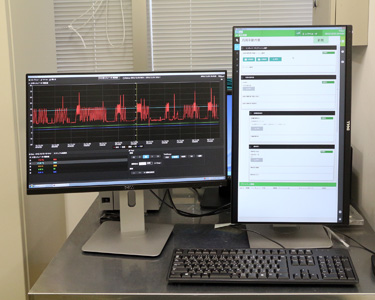
Manufacturing Management System

Standard Operating Procedure (SOP) Support System
Environmental Monitoring System (Facility/Factory)
Environmental Monitoring System (Isolator)
Cell Processing Facility/Factory Engineering Support
Validation Support to Ensure Regulatory Compliance as Required for Cell Processing
- INQUIRES
-
SHIBUYA CORPORATION
Regenerative Medicine Manufacturing Systems Div. Sales Dept.
2-1 Hokuyodai, Kanazawa, Ishikawa 920-0177 JAPAN
Telephone : +81(0)76-257-8915 Fax : +81(0)76-257-8919
E-mail rm-info@shibuya.co.jp

