REGENERATIVE MEDICINE MANUFACTURING SYSTEMS
Cell Processing Isolator “CPi”This new Isolator system paves the way for commercialization of regenerative medicine and cellular therapy without requiring a CPC production unit
A germ-free fully aseptic operational environment of a cell processing center (CPC) is recreated inside this isolator. The cells being cultured are completely separated from the technician creating a perfect environment for cell culture and cell processing.
This isolator based cell processing system provides a far safer environment for cell culture than biosafety cabinets or clean booth by eliminating risk from human contamination of cell cultures and cell processing, and widely used for various type od cells including iPS and stem cells.

- Humans, the primary contaminant source, are completely separated from cell culture and cell processing operation
- No need for a CPC facility which requires rigorous training and contamination control on cell cultures and cell processing
- An incubator for cell culture can be mounted and removed aseptically making the system suitable for mass cell cultivation
- Equipped with a receiving room and a pass box for both rapid transfer and extremely safe handling of material and cells
- Can also be used to provide a germ free aseptic environment for purposes other than cell cultures and cell processing
- Dramatic reduction in initial costs due to the elimination of the complex clean room zoning required for a CPC facility
- This purpose engineered unmanned and optimized aseptic environment significantly reduces running costs for cell cultures and cell processing
- Has a built-in HYDEC model vaporized hydrogen peroxide decontamination system for Isolator developed by unique Shibuya technology
- Realizes low running costs by reducing validation, energy consumption and general operating costs and reducing risk of product loss due to contamination
- Allows selection of a CPi operation pattern suitable for the utilization purpose

Incubator (Aseptically Connected to Isolator, VPHP Decontamination Available)
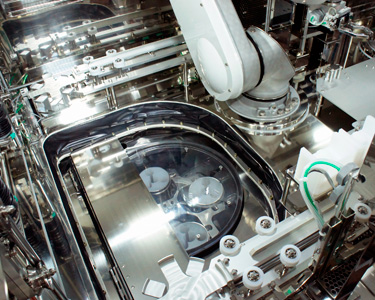
Built-in Centrifuge (VPHP Decontamination Available) for Cell Processing
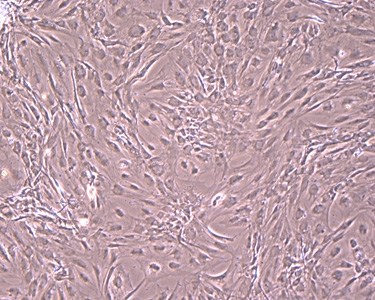
Cell Observation inside Isolators (Microscopic Image)

Dispensing and Capping Module
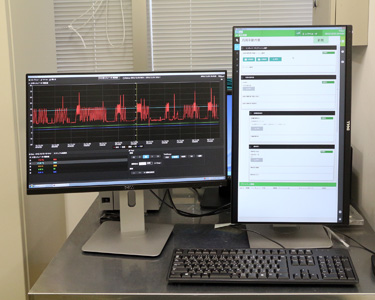
Manufacturing Management System

Standard Operating Procedure (SOP) Support System
Environmental Monitoring System (Facility/Factory)
Environmental Monitoring System (Isolator)
Remote Maintenance System
Cell Processing Facility/Factory Engineering Support
Validation Support to Ensure Regulatory Compliance as Required
- INQUIRES
-
SHIBUYA CORPORATION
Regenerative Medicine Manufacturing Systems Div. Sales Dept.
2-1 Hokuyodai, Kanazawa, Ishikawa 920-0177 JAPAN
Telephone : +81(0)76-257-8915 Fax : +81(0)76-257-8919
E-mail rm-info@shibuya.co.jp

